
Magnesium and calcium oxides are generally found in water pipes as precipitates.

Next, we notice that Na is unbalanced as there are three atoms on the right and only one on the left. So, we multiply the compound NaCl by 3 and obtain the following:įeCl 3 (aq.) + NaOH (aq.) → 3 NaCl (aq.) + Fe(OH) 3 (s/ppt.) We notice that there are three Cl atoms on the left-hand side of the equation and only one on the right. In order to balance the equation, we look at the Fe, Na, Cl, and OH –. Iron (III) chloride (FeCl 3) and sodium hydroxide (NaOH) react to form sodium chloride (NaCl) and insoluble iron (III) hydroxide (Fe(OH) 3).įeCl 3 (aq.) + NaOH (aq.) → NaCl (aq.) + Fe(OH) 3 (s/ppt.) Precipitation reaction can be balanced by ensuring that an equal number of atoms are present on both sides of the equation. Īll compounds of Li +, Na +, K +, Rb +, Cs +, and NH 4 +Ĭompounds of Li +, Na +, K +, Rb +, Cs +, and NH 4 +Ĭompounds of Li +, Na +, K +, Rb +, Cs +, NH 4 +, Sr 2+, and Ba 2+

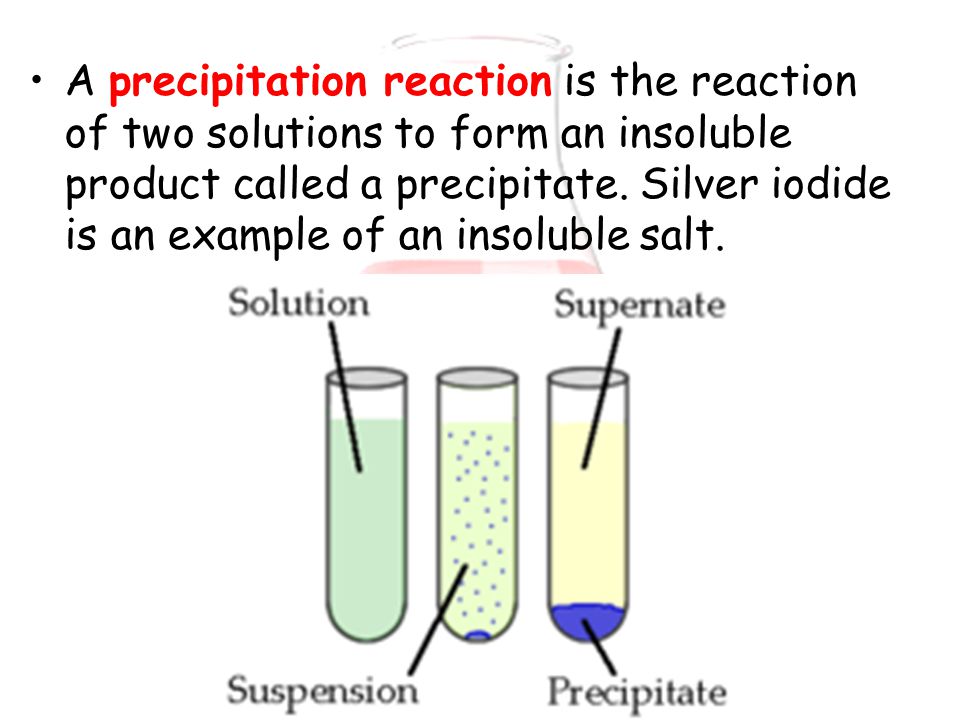
This table can be used to find and identify a precipitate. The following table shows the solubility of various compounds. If all the ions in a reaction are shown to be soluble, then no precipitation reaction occurs. If an ion is insoluble, then it forms a solid with an ion from the other reactant. According to this rule, if an ion is soluble, then it remains in its aqueous ion form. The fundamental rule used to tell whether a precipitate will form or not is the solubility rule.


 0 kommentar(er)
0 kommentar(er)
